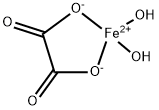
Ferrous Oxalate Dihydrate: Synthesis, Thermal Dehydration Kinetics & Multifunctional Applications
Dec 10,2025
Ferrous oxalate dihydrate is a coordination polymer that consists of chains of oxalate-bridged ferrous centers, each capped by water molecules. When heated, it dehydrates and decomposes into iron oxides and a pyrophoric black iron, and it also evolves carbon dioxide and carbon monoxide gases. Ferrous oxalate dihydrate is used for decorative glassware, as a pigment for plastics, paints, and lacquers, in the metal treatment industry, in photo developers' formulations, and the textile industry.

Synthesis of submicron ferrous oxalate using ferrous oxalate dihydrate
Ferrous oxalate dihydrate (FOD) can be used as a photo-Fenton catalyst with remarkable photo-Fenton catalytic and photocatalytic performances on organic pollutant degradation. Various reduction processes were compared in the current study to synthesize FODs from ferric oxalate solution utilizing the iron source in alumina waste red mud (RM), including natural light exposure (NL-FOD), UV light irradiation (UV-FOD), and hydroxylamine hydrochloride hydrothermal method (HA-FOD). The Ferrous oxalate dihydrates were characterized and employed as photo-Fenton catalysts for methylene blue (MB) degradation, and the effects of HA-FOD dosage, H2O2 dosage, MB concentration, and the initial pH were investigated. The results show that HA-FOD has submicron sizes and lower impurity contents with more rapid degradation rates and higher degradation efficiencies compared with the other two Ferrous oxalate dihydrate products.[1]
When using 0.1 g/L of each obtained FOD, 50 mg/L of MB can be rapidly degraded by HA- Ferrous oxalate dihydrate by 97.64% within 10 min with 20 mg/L of H2O2 at pH of 5.0, while NL-FOD and UV-FOD achieve 95.52% in 30 min and 96.72% in 15 min at the same conditions, respectively. Meanwhile, HA-FOD exhibits strong cyclic stability after two recycling experiments. Scavenger experiments reveal that the predominant reactive oxygen species responsible for MB degradation are hydroxyl radicals. These findings demonstrate that submicron Ferrous oxalate dihydrate catalyst can be synthesized using hydroxylamine hydrochloride hydrothermal process from ferric oxalate solution with high photo-Fenton degradation efficiency and reduced reaction time for wastewater treatment. The study also provides a new pathway of efficient utilization for RM.
Kinetic Modeling for Thermal Dehydration of Ferrous Oxalate Dihydrate Polymorphs
Thermal decomposition of solids is one of the most common steps during processing of a wide range of materials. The reaction mechanism and kinetics have long been studied in order to improve material syntheses and understand solid-state reactions. In this study, the thermal dehydration of ferrous oxalate dihydrate polymorphs was selected for the kinetic study because the experimental kinetic rate data under isothermal conditions indicate a sigmoidal mass-loss behavior after a long IP. The thermal dehydration process of ferrous oxalate dihydrate has been studied by many researchers in order to understand the synthetic process and control the morphology of iron(III) oxide polymorphs via this thermal decomposition process. We investigated the reaction kinetics through the formal kinetic analysis of the experimental mass-loss data recorded under different heating program modes and the morphological observations of the reacting particles. On the basis of the findings, we derived an overall kinetic model that integrates the kinetics of (1) the IP, (2) random nucleation on the surface, and (3) advancement of the reaction interface. The practical applicability of the kinetic model and its merits were demonstrated by comparing the results of kinetic simulations for the thermal dehydration of the two polymorph phases of ferrous oxalate dihydrate.[2]
The thermal dehydration of ferrous oxalate dihydrate polymorphs indicates significant IPs and the subsequent sigmoidal mass-loss trace under isothermal conditions. Thermal dehydration of square bipyramidal α-phase particles has longer IPs and higher thermal stability in comparison with the quadratic prismatic β-phase particles. The apparent activation energies for both IP and thermal dehydration of the α-phase are larger than those for the β-phase. In both samples, the reaction initiates on the surface with a distribution of the initiation time among the particles in the sample assemblage, which results in a distribution of the degree of conversion among the particles during the reaction. The sigmoidal shape of the mass-loss behavior is simulated satisfactorily, and the kinetic parameters for the three component processes are evaluated separately. The differences in the kinetic parameters for the IP and the PBR processes between the α- and β-phase samples conform to the kinetic behavior expected from the formal kinetic analysis of the reaction and the morphological observations. The kinetic simulation based on the combined model is also expected to be a possible method to evaluate the effects of specific reaction conditions to the respective component processes, for instance, the effect of atmospheric water vapor pressure on the thermal dehydration of ferrous oxalate dihydrate.
Ferrous oxalate dihydrate crystallization in simulated dihydrate phosphoric acid product
The basic fundamentals of ferrous oxalate dihydrate (FeC2O4.2H2O) crystallization including supersaturation, nucleation and crystal growth in simulated dihydrate phosphoric acid product with and without cetyl pyridinium chloride (CPC) additive were studied. Oxalic acid and ferrous sulfate heptahydrate crystals were mixed with dilute phosphoric acid (28% P2O5) at 60 °C and the turbidity of the reaction mixture was measured at different time intervals. Induction time of ferrous oxalate dihydrate crystals was calculated at different supersaturation ratios ranging from 2.5 to 6.7. With increasing the supersaturation ratio, the induction time decreased. The nucleation rates are 46.4 × 1028 nuclei cm−3 s−1 and 50.2 × 1028 nuclei cm−3 s−1 at supersaturation ratio 6.7 with and without CPC addition, respectively. The surface energy increases with CPC addition compared to the baseline. In addition, the formed crystals are modified from cubic shape to rod-like shape with increasing CPC dose.[3]
References
[1]Yang, Yuxin et al. “Synthesis of submicron ferrous oxalate from red mud with high Fenton catalytic performance on degradation of methylene blue.” Environmental science and pollution research international vol. 30,36 (2023): 85210-85222. doi:10.1007/s11356-023-28308-z
[2]Ogasawara, Haruka, and Nobuyoshi Koga. “Kinetic modeling for thermal dehydration of ferrous oxalate dihydrate polymorphs: a combined model for induction period-surface reaction-phase boundary reaction.” The journal of physical chemistry. A vol. 118,13 (2014): 2401-12. doi:10.1021/jp500619q
[3]Abdel-Ghafar, H M et al. “Innovative findings about ferrous oxalate dihydrate crystallization in simulated dihydrate phosphoric acid product.” Water science and technology : a journal of the International Association on Water Pollution Research vol. 77,11-12 (2018): 2940-2945. doi:10.2166/wst.2018.294
- Related articles
- Related Qustion
2-Bromopyridine enables MAPA reagents and polyheteroarylated 2-pyridones synthesis via copper-catalyzed cross-coupling, with broad synthetic utility.....
Dec 10,2025Organic reagentsFerrous oxalate dihydrate
6047-25-2You may like
Ferrous oxalate dihydrate manufacturers
- Ferrous oxalate dihydrate
-
- 2025-12-11
- CAS:6047-25-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Ferrous oxalate dihydrate
-

- $10.00 / 1kg
- 2025-12-11
- CAS:6047-25-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Ferrous oxalate dihydrate
-

- $0.00 / 1KG
- 2025-06-27
- CAS:6047-25-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg